FDA Issues Its Largest-Ever Clearance for a Family of 3D-Printed Lumbar Fusion Devices
Osseus Fusion Systems announced FDA 510(k) clearance for Aries, its family of 3D-printed lumbar interbody fusion devices. Aries is one of the most comprehensive families of 3D-printed products in the spine and orthopedic industry, with implants available for lateral (LLIF), anterior (ALIF), straight and curved transforaminal (TLIF) procedures, as well as an additional surgeon-inspired implant designed for oblique (OLIF) procedures using the Kambin’s Triangle approach. The approval is one of the largest FDA 510(k) clearance for an entire family of 3D-printed lumbar interbody fusion devices at once in the history of the spine industry.
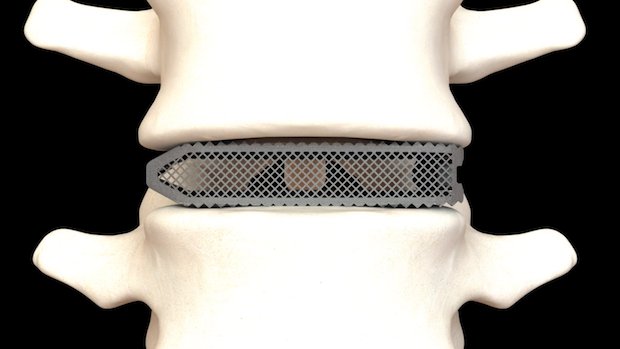
Aries devices are constructed from highly porous titanium material, which is optimized for bone fusion and biological fixation using PL3XUS, Osseus’ innovative and proprietary 3D-printing technology. With upwards of 80% porosity, Aries devices are among the most porous 3D-printed spinal implants available.
“We are thrilled to launch the Aries family of 3D-printed lumbar interbodies,” says Eric Hansen, Co-Founder and CEO of Osseus. “The clinical benefits of 3D-printed titanium speak for themselves and Osseus is poised to capture market share in an exponentially growing industry like never before. Osseus is unique in our ability to bring surgeon-inspired implants to market quicker than any competitor, and our focus on R&D delivers unparalleled performance and quality to our surgeons and patients.”
The Aries family of lumbar interbody fusion devices features a proprietary mesh lattice structure, which helps reduce the stiffness of the cage and maximize bone graft packability. The distinctive mesh structure is optimized to create a superior environment for bone cell fixation and proliferation. Each Aries lumbar interbody fusion device comes in a wide variety of footprints, heights, and lordotic angles, to adapt to a variety of patient anatomies.
“As a surgeon, it’s very exciting to participate in the device development process and see your ideas brought to life so quickly,” says Dr. Sam Joseph, Jr., who collaborated with the Osseus engineering team. “Using 3D printing, we were able to go from design, to prototype, to finished product much faster than with traditional manufacturing—which means patients get access to more advanced treatments sooner, too.”
This is the fourth FDA 510(k) clearance that Osseus has received, effectively doubling the company’s product portfolio.
Source: SPINEMARKET Group




Recent Comments