CoreLink Wins FDA Clearance for Standalone Cervical Spine System
CoreLink announced on June 30 that it received FDA 510(k) clearance for its F3D-C2 standalone cervical system for ACDF procedures.
The F3D-C2 standalone cervical device eliminates the need for a supplemental fixation plate to make ACDF (anterior cervical discectomy and fusion) procedures easier to complete and a faster process overall.
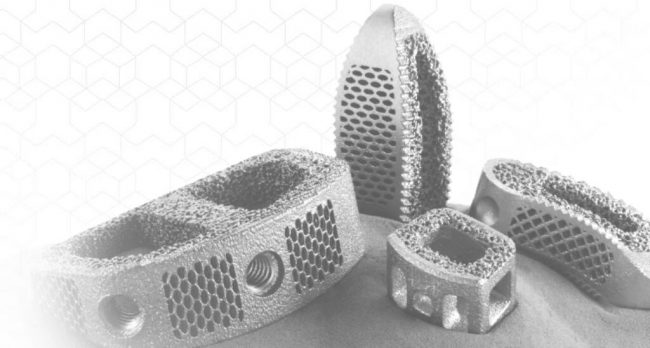 Image via CoreLink
Image via CoreLink
St. Louis-based CoreLink’s system includes its 3D-printed Mimetic Metal technology, incorporated into the spacer to emulate key characteristics of natural bone and provide lower stiffness than machined titaium while minimizing imaging artifact.
The company said in a news release that the F3D-C2 system will be available in multiple footprints, heights and lordotic angles.
“The F3D-C2 standalone cervical system features the only 3D-printed spine technology with both a trabecular structure and directional support lattices designed to allow for fusion throughout the entire implant,” CoreLink CEO Jay Bartling said in the release. “Despite the challenges that have occurred in our industry recently, we have continued aggressively investing in new product development. We are committed to growing with our surgeon and distributor partners and expect several additional product launches this year.”
Source: CoreLink




Recent Comments