CoreLink Wins FDA Clearance for Standalone Cervical Spine System
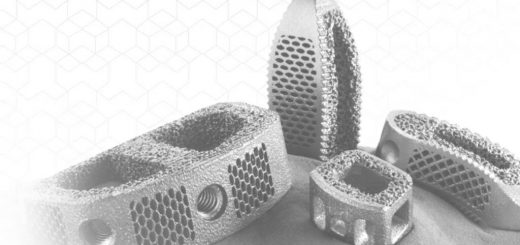
CoreLink announced on June 30 that it received FDA 510(k) clearance for its F3D-C2 standalone cervical system for ACDF procedures. The F3D-C2 standalone cervical device eliminates the need for a supplemental fixation plate to...


Recent Comments