CSIRO is Developing 3D Printed Self-Expanding Stents
CSIRO is developing a new manufacturing technology for producing the world’s first customised 3D printed self-expanding stents.
![]() The challenge -A need for customised self-expanding stents
The challenge -A need for customised self-expanding stents
Peripheral Arterial Disease (PAD) is a progressive disease caused by the build up of plaque in the arterial system that carries blood to the arms, legs and vital organs. Approximately ten percent of the world’s population has PAD. Endovascular stenting is commonly used in PAD patients because it’s minimally invasive and has shorter recovery times.
There are currently two types of metallic stents – balloon-expandable stents made from stainless steel or cobalt-chromium alloys, and self-expanding stents made from nitinol, a nickel-titanium alloy. Both are off-the-shelf products. Surgeons can select from various shapes and sizes of the tiny mesh tubes to suit their patients, but overall they are limited by the stents available.
CSIRO was contacted by an experienced vascular surgeon who has treated thousands of patients with vascular disorders, about the need for an improved product for treating patients. An opportunity for developing a self-expanding stent that could be customised to suit individual patients was identified.
![]() Response from CSIRO – Working with industry to create an exciting new product
Response from CSIRO – Working with industry to create an exciting new product
The specialist researchers in CSIRO’s Lab22 have built many world first biomedical parts. CSIRO worked with industry partners to make the first 3D printed titanium heel and the first 3D printed titanium sternum and partial rib cage. So when Medical Innovation Hub (MIH) approached CSIRO about making the world’s first 3D printed nitinol stent, CSIRO was open to the challenge.
CSIRO’s team has been exploring the feasibility of 3D printing nitinol self-expanding stents using an additive manufacturing process called Selective Laser Melting (SLM). SLM can’t be matched by other manufacturing methods. It creates complex products with high geometric accuracy while allowing the design freedom to produce patient-specific parts. But using SLM to develop a nitinol stent was not without its challenges.
Nitinol is a shape memory alloy that displays superelasticity when stressed. Its unique properties come from its crystal structure that can change when stressed or heated. The alloy’s two distinct phases (martensite and austenite) are determined by temperature, and the phase transformation temperatures are extremely sensitive to the stent manufacturing conditions. For the stent to display self-expansion, the transformation temperature needs to be below the body temperature, 37°C.
The SLM parameters had to be suitably selected to fabricate the ultra-fine mesh structure of the stents, comprising fine struts 80-200 µm in size. They also needed to be fine-tuned so that the desired phase transformation temperature, below 37°C, could be achieved in the finished stent.
![]() The results – A better patient experience and a superior product
The results – A better patient experience and a superior product
Using additive manufacturing technologies, clinically certified bespoke 3D printed nitinol self-expanding stents can potentially be manufactured on site, under the surgeon’s direction.
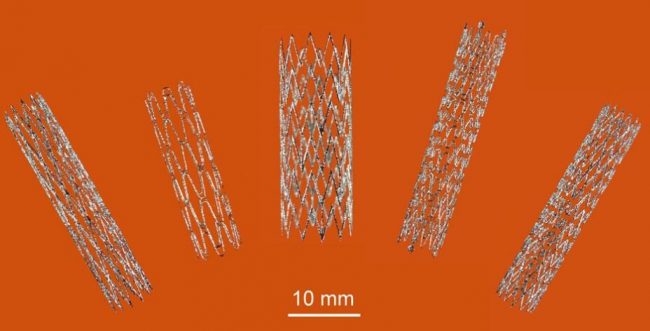 3D printed self-expanding nitinol stents, manufactured using a Selective Laser Melting (SLM) process in our Lab22 in Melbourne. Image via CSIRO.
3D printed self-expanding nitinol stents, manufactured using a Selective Laser Melting (SLM) process in our Lab22 in Melbourne. Image via CSIRO.
The design freedoms offered by additive manufacturing not only allow for the customisation of these stents to specific size of vessel proximal and distal diameters, but can also accommodate larger sized stents, cross branches and new shapes for proximal and distal vessels.
Working with MIH to create this exciting new product is another way that we’re supporting Australia’s Biomedical Manufacturing industry. 3D printed nitinol stents offer the potential for customisation, inventory reduction (less inventory on shelf) and resource efficiencies. For the patient, these special stents provide a better fitting device, better conformity to blood vessels and improved recovery time – equating to an improved patient experience.
Source: CSIRO


Recent Comments