3-D Printed Implants Show Promise For Treating Spinal Cord Injury
3-D printed implants could one day help restore neural connections and lost motor function in patients with spinal cord injury. The implants, developed by engineers and neuroscientists at the University of California, San Diego, are soft bridges that guide new nerve cells to grow across the site at which the spinal cord has been severed. The work has so far shown promise in rats with severe spinal cord injury.
Researchers published their work in the Jan. 14 issue of Nature Medicine.
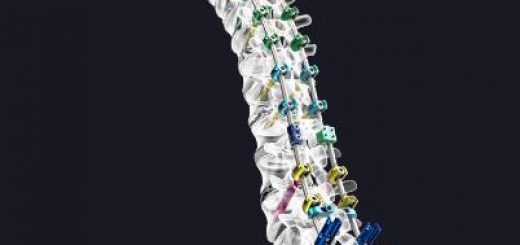
 This 3-D printed hydrogel implant, modeled from MRI scans, has a design customized to fit a patient’s spinal cord injury.
This 3-D printed hydrogel implant, modeled from MRI scans, has a design customized to fit a patient’s spinal cord injury.
The implants are hydrogel structures that can be rapidly 3-D printed into different sizes and shapes, making them easily customizable to fit the precise anatomy of a patient’s spinal cord injury. Researchers fill the implants with neural stem cells and then they are fitted, like missing puzzle pieces, into sites of spinal cord injury. New nerve cells grow and axons — long, hair-like extensions through which nerve cells pass signals to other nerve cells — regenerate, allowing new nerve cells to connect with each other and the host spinal cord tissue.
“Using our rapid 3-D printing technology, we’ve created a scaffold that mimics central nervous system structures. Like a bridge, it aligns regenerating axons from one end of the spinal cord injury to the other. Axons by themselves can diffuse and regrow in any direction, but the scaffold keeps axons in order, guiding them to grow in the right direction to complete the spinal cord connection,” said co-senior author Shaochen Chen, professor of nanoengineering at the UC San Diego Jacobs School of Engineering and faculty member of the Institute of Engineering in Medicine at UC San Diego.
3-D bioprinting experts in Chen’s lab teamed up with neural stem cell biology experts in the lab of Mark Tuszynski, professor of neuroscience and director of the UC San Diego Translational Neuroscience Institute, who is also co-senior author on the study.
“In recent years and papers, we’ve progressively moved closer to the goal of abundant, long-distance regeneration of injured axons in spinal cord injury, which is fundamental to any true restoration of physical function,” said Tuszynski.
“The new work puts us even closer to the real thing because the 3D scaffolding recapitulates the slender, bundled arrays of axons in the spinal cord. It helps organize regenerating axons to replicate the anatomy of the pre-injured spinal cord,” added co-first author Kobi Koffler, assistant project scientist in Tuszynski’s group.
![]() Faster, more precise printing
Faster, more precise printing
 A larger (four-centimeter) implant modeled to fit an actual human spinal cord injury.
A larger (four-centimeter) implant modeled to fit an actual human spinal cord injury.
Credit: UC San Diego
The implants contain dozens of tiny, 200-micrometer-wide channels (twice the width of a human hair) that guide neural stem cell and axon growth along the length of the spinal cord injury. The rapid 3-D printing technology that Chen’s team developed produces two-millimeter-sized implants in just 1.6 seconds. Traditional nozzle printers take several hours to produce much simpler structures.
And the process is scalable to human spinal cord sizes. As a proof of concept, researchers printed four-centimeter-sized implants modeled from MRI scans of actual human spinal cord injuries. These were printed within 10 minutes.
“This shows the flexibility of our 3-D printing technology. We can quickly print out an implant that’s just right to match the injured site of the host spinal cord regardless of the size and shape,” said co-first author Wei Zhu, nanoengineering postdoctoral fellow in Chen’s group.
![]() Restoring lost connections
Restoring lost connections
 A larger (four-centimeter) implant modeled to fit an actual human spinal cord injury.
A larger (four-centimeter) implant modeled to fit an actual human spinal cord injury.
Credit: UC San Diego
Researchers grafted two-millimeter implants, loaded with neural stem cells, into sites of severe spinal cord injury in rats. After a few months, new spinal cord tissue had regrown completely across the injury and connected the severed ends of the host spinal cord. Treated rats regained significant functional motor improvement in their hind legs.
“This marks another key step toward conducting clinical trials to repair spinal cord injuries in people,” Koffler said. “The scaffolding provides a stable, physical structure that supports consistent engraftment and survival of neural stem cells. It seems to shield grafted stem cells from the often toxic, inflammatory environment of a spinal cord injury and helps guide axons through the lesion site completely.”
Additionally, the circulatory systems of the treated rats had penetrated inside the implants to form functioning networks of blood vessels, which helped the neural stem cells survive. “Vascularization is one of the main obstacles in engineering tissue implants that can last in the body for a long time,” Zhu said. “3-D printed tissues need vasculature to get enough nutrition and discharge waste. Our group has done work on 3-D printed blood vessel networks before, but we didn’t include it in this work. Biology just naturally took care of it for us due to the excellent biocompatibility of our 3-D scaffolds.”
![]() Neural stem cell biology meets 3-D bioprinting
Neural stem cell biology meets 3-D bioprinting
This advance marks the intersection of two longstanding lines of work at the UC San Diego School of Medicine and the Jacobs School of Engineering.
For more than a decade, Tuszynksi and colleagues have steadily progressed toward the ultimate goal of restoring function in people after a severe spinal cord injury. Previous published research, based on stem cell-based therapies, established the concept and potential, first in rats and later in monkeys. Along the way, they also upended dogma about how neurons grow.
Earlier this year, his team created a new line of spinal cord neural stem cells capable of dispersing throughout the spinal cord. The cells can be maintained for long periods of time, constituting a potentially improved, clinically translatable cell source for future replacement strategies.
Meanwhile on the engineering side, Chen’s team has been developing a next-generation 3-D bioprinting technology capable of producing detailed microstructures that mimic the sophisticated designs and functions of biological tissues. Chen’s lab has used this technology in the past to create life-like liver tissue and intricate blood vessel networks. One of their ongoing projects involves printing heart tissue for people who have suffered a heart attack and for treating other cardiac diseases.
The new spinal cord implant is one of the latest in this line of 3-D bioprinted tissues.
“Biological validation is what makes this work more powerful,” Chen said. “No matter how beautiful the engineering of an implant, to make it useful you need to work with biologists and medical experts. Collaborating with groups at the UC San Diego School of Medicine has proven to be invaluable to our projects.”
![]() Next steps
Next steps
The researchers are currently scaling up the technology and testing on larger animal models in preparation for potential human testing. Next steps also include incorporation of proteins within the spinal cord scaffolds that further stimulate stem cell survival and axon outgrowth.
Chen and Zhu have co-founded the startup, Allegro 3-D, to commercialize their rapid bioprinting technology.
Source: University of California


Recent Comments