Simulation Makes Personalized Healthcare and 3D Printed Implants Possible
We all understand that no two people are alike. However, when determining treatments, the medical profession tends to treat all patients based on an average body type. The truth is, personalized healthcare can help improve a patient’s chance of survival, recovery time and quality of life.
To that end, VOLMO, an ANSYS Startup Program member, has developed the ImageSim software. This medical software processes medical scans so that the geometry of 3D printed implants, stents and fixation plates can be digitally designed.
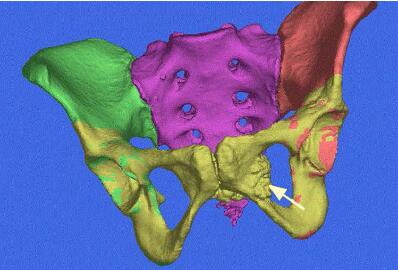 A patient’s computed tomography (CT) scan data is imported into VOLMO’s ImageSim software. Doctors can clearly see the cancerous region on the pelvis and plan accordingly.
A patient’s computed tomography (CT) scan data is imported into VOLMO’s ImageSim software. Doctors can clearly see the cancerous region on the pelvis and plan accordingly.
VOLMO then tests the geometry of the 3D printed implants in ANSYS Mechanical. Once these tests are complete, VOLMO prints the implants using additive manufacturing.
Instead of hoping for the best and preparing for the worst, medical practitioners can use ImageSim to plan treatments, practice surgeries and optimize patient recovery. This personalized healthcare strategy improves the ability of medical practitioners to get their patients back on their feet quickly and safely.
![]() In Silico Trials, Via ANSYS Simulation, Enable Personalized Healthcare
In Silico Trials, Via ANSYS Simulation, Enable Personalized Healthcare
One of the benefits of VOLMO’s brand of personalized healthcare is that it can digitally test (in silico test) a patient’s treatment before surgery.
Instead of picking a treatment that was tested on clinical trials of selected patients, medical practitioners can design hundreds, perhaps thousands, of treatments that are simulated on the digital twin of the patient.
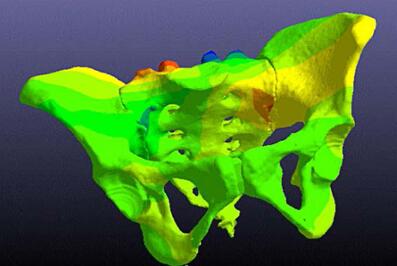 A personalized pelvic implant is created using VOLMO’s ImageSim software. It is then tested using ANSYS Mechanical.
A personalized pelvic implant is created using VOLMO’s ImageSim software. It is then tested using ANSYS Mechanical.
These in silico trials, using Mechanical simulations, will insure that the 3D printed implants will not cause any stress or deformation under loading conditions that are specific to the patient.
Once these simulations are completed, VOLMO can print as many implants as it needs to perform physical tests if necessary. Once these final rounds of testing are completed, a final implant can be printed that is ready for surgery.
VOLMO’s in silico trials can expediate the approval processes of the U.S. Food and Drug Administration (FDA) and other regulatory bodies. Additionally, VOLMO’s technology can help close the door on one-size-fits-all medicine and make personalized healthcare a reality.
Source: ANSYS



Recent Comments